Request a Tool
Accurate Buffer pH Calculator-Simplify Your Chemistry Calculations
Use the Buffer pH Calculator to quickly and accurately determine the pH of a solution containing a weak acid and its conjugate base.
Input
Output
Formula

- Ka = Acid dissociation constant
- [A] = Concentration of conjugate base
- [HA] = Concentration of the acid A
Use the upper given formula for manual calculations. No sign-up, registration OR captcha is required to use this tool.
Input
Output
Formula
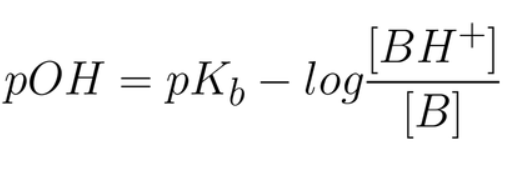
- Kb = Base dissociation constant
- [B] =Concentration of the weak base (B)
- [HB+] = Concentration of the conjugate acid
Use the upper given formula for manual calculations. No sign-up, registration OR captcha is required to use this tool.
What is the buffer ph?
A buffer pH is the pH of a buffer solution which is created to further marginals the change of pH when an amount of acid or base is added. These solutions are formed through the reaction of a weak acid with a linked weak conjugate base or weak base with a linked weak conjugate acid. Buffer solutions are important when carrying out chemical reactions or working with biological systems and even in engineering processes. For instance pH maintainers in human body control and maintain a balance of acidity level for intercellular functioning. Buffer pH depends on the concentration and characteristic of its contents and is vital in procedures that need an efficient pH control.
What is a buffer ph calculator?
A buffer pH calculator is an online tool which is used to calculate the pH of the buffer solution. A buffer solution is made up of either an antigen acid and its conjugate base or a genewite base and its conjugate acid. These solutions do not show changes in pH when small portions of acids or bases are added, thereby being useful in laboratory experiments, biological processes as well as industries.
The buffer’s pH, given the required values, is easily calculated by the advanced calculator. This is particularly so in laboratories, preparation of pharmaceutical products and in environmental management where pH is critical in influencing the stability of certain products for use. The buffer pH calculator is effective and efficient to save time and avoid error to provide a proper method to handle pH sensitive environments.
Why Use a buffer ph calculator tool
Accuracy in Calculations
Safeguards the pH computations thereby minimizing human interference and probable errors during computation.
Saves Time
Able to rapidly solve pH issues especially where they are used in cases such as in laboratories or industrial processes.
Simplifies Complex Processes
Can manage the Henderson-Hasselbalch equation and other arithmetic operations in an easy way.
User-Friendly
User friendly interface that can easily be used by any user without major understanding of computer operations.
Supports Learning
Help students and researchers to explain the behavior of the buffer system.
Essential for Experiments
Cooperates with keeping pH balance during the experiments when precise results are necessary.
Adaptable for Various Applications
Applicable in industries such as biochemical, environmental, and even pharmaceutical industries.
Where Can a buffer ph calculator tool be used
Laboratories
Common in chemical and biological labs where solutions of buffers are required for an experiment and researchers to prepare and maintain buffer solutions with precise pH levels for experiments.
Biotechnology
Helps maintain optimal pH conditions in biological processes, such as enzyme reactions and cell cultures.
Environmental Science
Used to analyze and maintain pH levels in water samples during pollution studies or aquatic ecosystem assessments.
Education and Academia
A valuable tool for students and teachers to calculate and understand buffer systems in chemistry lessons and practicals.
Healthcare
Used in diagnostic labs to prepare buffers for tests and analyses involving biological samples.
Cosmetic Industry
Helps in formulating skincare and cosmetic products with the correct pH to avoid irritation and ensure product stability.
How to Use a buffer ph calculator tool
Using a Buffer ph Calculator is straightforward and involves these simple steps:
Select the Buffer Type
Choose the type of buffer solution you are working with, such as PH Acidic Buffer and Ph Alkaline Buffer.
Enter the Concentration of Components
Input the molar concentration of the weak acid (or base) and its conjugate base (or acid) in the solution.
Provide the pKa or pKb Value
Enter the dissociation constant (pKa for weak acids or pKb for weak bases) of the buffering compound.
Click Calculate
Once you input the values this tool automatically determines the pH of the buffer solution.
Review the pH Result
The calculator will display the pH value of the buffer based on your inputs, helping you ensure it meets your needs.
Clear Button
This button that allows you to clear the input fields and start a new calculation. This button is helpful when you need to perform multiple time calculations or make changes to the input values.
Conclusion
Therefore, it is important and quite logical to focus on the so-called buffer pH and calculations to preserve the stability of solutions in diversified chemical and biological processes. It is also possible to predict the behavior of the buffer with reference to its pH, based on the operation of the buffer equation and the concentration of the acid and conjugate base components. It is common knowledge in industries like the pharmaceutical industry or environmental science or in laboratories to guarantee the right conditions for reactions or biological processes. A reliable buffer pH calculation supports accurate control and improves the efficiency of the buffer in different real-life operations.